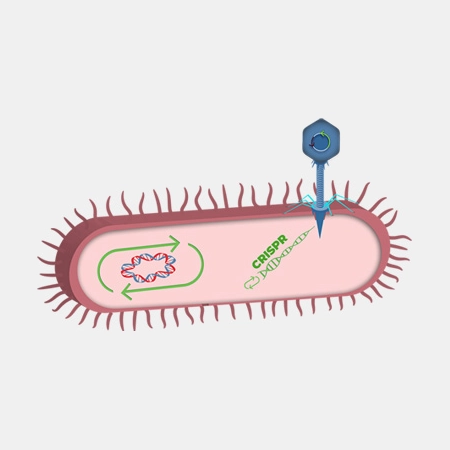
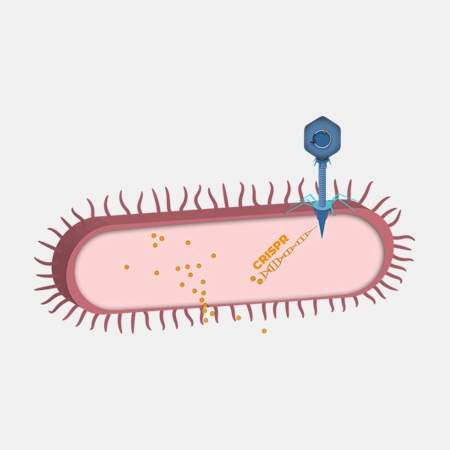
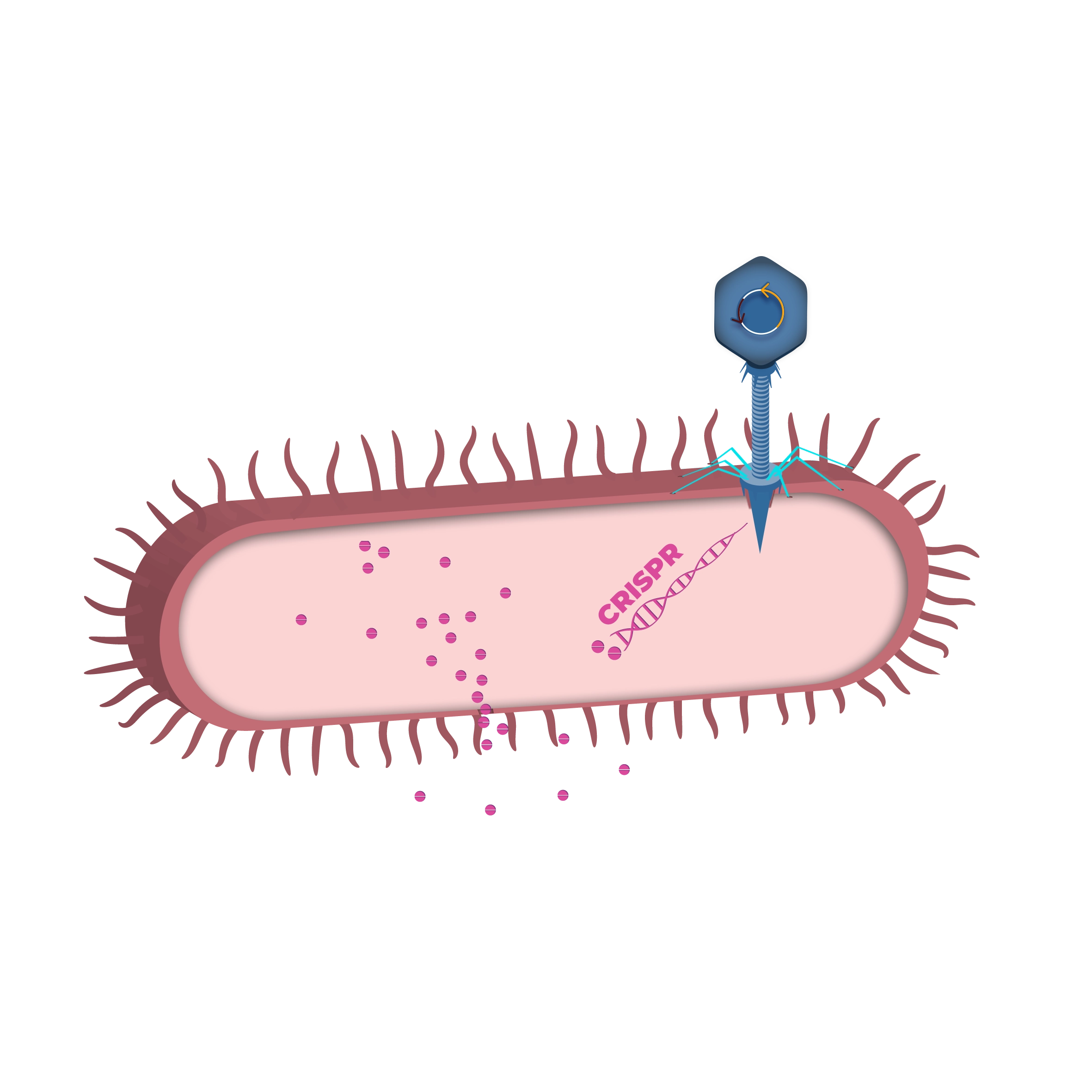
TBX 201
TBX201 is designed to reduce the incidence and severity of severe diarrhea caused by irinotecan. Irinotecan is a first-line chemotherapy drug for the treatment of several metastatic cancer indications. Clinical use of irinotecan in over 100,000 patients annually is associated with life-threatening toxicity and treatment interruptions for many patients.